Precision medicine has emerged as a transformative approach in cancer treatment, revolutionizing how therapies are tailored to individual patients. This method utilizes detailed genetic, environmental, and lifestyle information to design personalized treatment plans, offering a more targeted and effective strategy against cancer. Recent advancements in this field have significantly impacted patient outcomes and are reshaping the landscape of oncology.
One of the most significant strides in precision medicine is the development of targeted therapies. These treatments focus on specific genetic mutations and molecular pathways that drive cancer growth. For instance, the introduction of targeted drugs such as osimertinib (Tagrisso) has dramatically improved outcomes for patients with non-small cell lung cancer (NSCLC) who have mutations in the EGFR gene. Clinical trials have shown that osimertinib can reduce tumor size by 70% in patients with EGFR mutations, with a progression-free survival rate extending to 18.9 months compared to 10.2 months with older therapies.
Another breakthrough is the advancement of genomic profiling, which allows for the comprehensive analysis of a tumor’s genetic makeup. Programs like the Cancer Genome Atlas (TCGA) have provided invaluable data, revealing that approximately 60% of cancers are driven by specific genetic mutations. This has led to the development of therapies targeting these mutations, such as the BRAF inhibitors used for melanoma. Studies indicate that patients with BRAF V600E mutations in melanoma show a 50% improvement in overall survival when treated with BRAF inhibitors compared to standard chemotherapy.
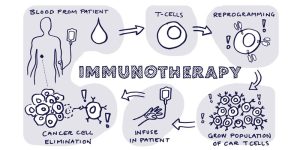
Immunotherapy, a cornerstone of modern precision medicine, has also seen remarkable progress. CAR-T cell therapy, which involves engineering a patient’s T-cells to target cancer cells, has shown impressive results in hematologic cancers. For example, Kymriah, the first FDA-approved CAR-T therapy, has achieved a remission rate of 83% in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL). This contrasts sharply with the 10% remission rate seen with conventional treatments.
The integration of precision medicine into routine clinical practice is being driven by both technological advancements and increasing accessibility. Next-generation sequencing (NGS) technology has become more affordable, enabling broader use of genomic profiling in cancer diagnosis and treatment. The cost of NGS has decreased from $10 million for a single genome in 2007 to around $1,000 today, making comprehensive genomic analysis more accessible to patients and healthcare providers.
Despite these advancements, challenges remain in the implementation of precision medicine. Variability in tumor biology and the emergence of resistance to targeted therapies can complicate treatment. Additionally, disparities in access to advanced therapies and genomic testing highlight the need for equitable healthcare solutions. For instance, while precision medicine offers tremendous potential, access to cutting-edge treatments and genomic testing is often limited to well-funded institutions, leaving some patients with less access to these innovations.
In conclusion, precision medicine is reshaping the field of oncology, offering more personalized and effective treatment options for cancer patients. The latest advancements in targeted therapies, genomic profiling, and immunotherapy are providing hope and improving outcomes for many. As the field continues to evolve, addressing challenges such as access and resistance will be crucial in ensuring that the benefits of precision medicine are available to all patients.